 KCET Chemistry Answer Key 2024 Unofficial (Image credit: Pexels)
KCET Chemistry Answer Key 2024 Unofficial (Image credit: Pexels)KCET Chemistry Answer Key 2024: Chemistry is one of the compulsory papers that the candidates must appear for the KCET 2024 exam, whether they are from the PCM or PCB stream. KCET Chemistry question paper 2024 consists of 60 questions for 60 marks and there is no negative marking for wrong or unattempted questions. Here, the candidates can check out KCET Chemistry unofficial answer key 2024 as prepared by our subject experts. Note that the Official KCET Answer Key 2024 Release Date is expected shortly after the exam conclusion. Until then, the candidates can refer to the unofficial answer key to calculate their tentative obtainable score. In Day 1's shifts, out-of-syllabus questions were asked, check the report here: KCET 2024 Out-of-Syllabus Questions Asked: 17 from Mathematics, 10 from Biology.
Also Read | Provisional KCET Answer Key 2024 Released: Download PDF
| IMPORTANT NOTE: |
|---|
| In all KCET paper sets, all questions are the same, only their order is shuffled. Hence, direct questions and their answers are provided here instead of the question number and answer number for each separate set. Students of all question paper sets can go through the following answer key to crosscheck their answers. |
KCET Chemistry Answer Key 2024 Unofficial (All Sets)
The questions in all sets of Chemistry question papers of KCET 2024 will remain the same but the question number or sequence will change. Therefore, the question-wise answer key is being provided here so that all candidates can find answers to the questions asked. You can also check KCET Chemistry Question Paper Analysis 2024.
| Sr. No. | Questions | Answers |
|---|---|---|
| 1 | Propanone and Propanal are: | Functional isomers |
| 2 | Sodium ethanoate on heating with soda lime gives 'X'. Electrolysis of aqueous solution of sodium ethanoate gives 'Y'. 'X' and 'Y' respectively are: | Methane and Ethane |
| 3 | But-1-yne on reaction with dil. H2SO4 in the presence of Hg2+ ions at 333 K gives : | 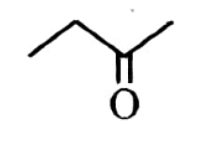 |
| 4 | Biologically active adrenaline and ephedrine used to increase blood pressure contain: | Secondary amino group |
| 5 | In the reaction Aniline → (in presence of NaNO2, dil.HCl) → P → (in presence of Phenol, NaOH) → Q 'Q' is : | para-hydroxyazobenzene |
| 6 | The female sex hormone which is responsible for the development of secondary female characteristics and participates in the control of menstrual cycle is : | Estradiol |
| 7 | The type of linkage present between nucleotides is : | Phosphodiester linkage |
| 8 | α - D - (+) - glucose and β - D - (+) - glucose are : | Anomers |
| 9 | Which of the following set of polymers are used as fibre ? (i) Teflon (ii) Starch (iii) Terylene (iv) Orlon | (iii) and (iv) |
| 10 | The biodegradable polymer obtained by polymerisation of Glycine and Aminocaproic acid is: | Nylon 2 - Nylon 6 |
| 11 | The compound | Saccharin |
| 12 | Which one of the following is a cationic detergent? | Cetyltrimethylammonium bromide |
| 13 | For which one of the following mixtures is composition uniform throughout? | Dilute aqueous solution of sugar |
| 14 | The energy associated with first orbit of He+ is : | - 8.72 × 10-18 J |
| 15 | A metalloid is : | Sb |
| 16 | A pair of isoelectronic species having bond order of one is : | O22-, F2 |
| 17 | Iderntify the wrong relation for real gases : | Z = Videal / Vreal |
| 18 | From the diagram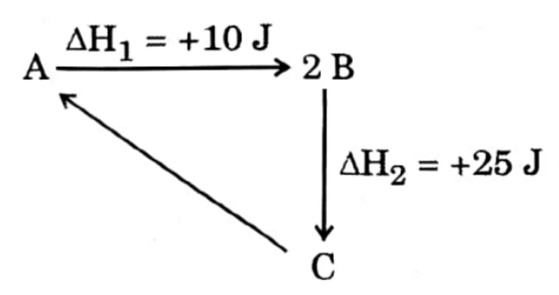 △rH for the reaction C → A is : | -35 J |
| 19 | In the analysis of III group basic radicals of salts, the purpose of adding NH4Cl(s) to NH4OH is: | to suppress the dissociation of NH4OH |
| 20 | Solubility product of CaC2O4 at a given temperature in pure water is 4 × 10-9 (mol L-1)2. Solubility of CaC2O4 at the same temperature is : | 6.3 × 10-4 mol L-1 |
| 21 | In the reaction between moist SO2 and acidified permanganate solution : | SO2 is oxidised to SO42- MnO4- is reduced to Mn2+ |
| 22 | Which one of the following properties is generally not applicable to ionic hydrides? | Volatile |
| 23 | Which one of the following nitrate will decompose to give NO2 on heating? | LiNO3 |
| 24 | Which of the following halides cannot be hydrolysed? | CCl4 |
| 25 | 0.48 g of an organic compound on complete combustion produced 0.22 g of CO. The percentage of C in the given organic compound is : | 12.5 |
| 26 | In the given sequence of reactions, identify 'P', 'Q', 'R' and 'S' respectively.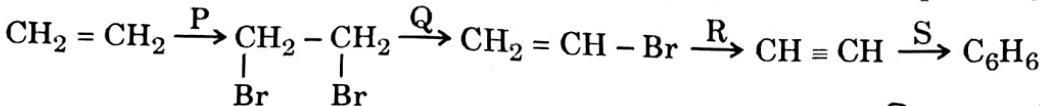 | Br, Alc. KOH, NaNH, Red hot iron tube |
| 27 | The first chlorinated organic insecticide prepared is: | DDT |
| 28 | Which of the following crystals has the unit cell such that a = b ≠ c and α = β = 90°, γ = 120° ? | Cinnabar |
| 29 | MnO exhibits : | Antiferromagnetism |
| 30 | The number of atoms in 4.5 g of a face-centred cubic crystal with edge length 300 pm is : (Given density = 10 g cm-3 and NA = 6.022 × 1023) | 6.6 × 1022 |
| 31 | Vapour pressure of a solution containing 18 g of glucose and 178.2 g of water at 100°C is : (Vapour pressure of pure water at 100°C = 760 torr) | 752.4 torr |
| 32 | A mixture of phenol and aniline shows a negative deviation from Raoult's law. This is due to the formation of: | Intermolecular Hydrogen bond |
| 33 | Which one of the following pairs will show positive deviation fron Raoult's Law? | Benzene - Methanol |
| 34 | How many Coulombs are required to oxidise 0.1 mole of H2O to oxygen ? | 1-93 × 104 C |
| 35 | A current of 3 A is passed through a molten calcium salt for 1 hr 47 min 13 sec. The mass of calcium deposited is: (Molar mass of Ca = 40 g mol-1 ) | 4.0 g |
| 36 | The value of 'A' in the equation λm = λm0 - A√C is same for the pair : | NaCl and KBr |
| 37 | For the reaction, AB, Ea = 50 kJ mol-1 and △H = - 20 kJ mol-1. When a catalyst is added, Ea decreases by 10 kJ mol-1. What is the Ea for the backward reaction in the presence of catalyst? | 60 kJ mol-1 |
| 38 | For the reaction PCl5 → PCl3 + Cl2, rate and rate constant are 1.02 × 10-4 mol L-1 s-1 and 3.4 × 10-5 s-1 respectively at a given instant. The molar concentration of PCl5 at that instant is: | 3.0 mol L-1 |
| 39 | Which one of the following does not represent Arrhenius equation? | k= AeEa/RT |
| 40 | Identify the incorrect statement: | Brownian movement is due to balanced bombardment of molecules of dispersion medium on colloidal particles. |
| 41 | For the coagulation of positively charged hydrated ferric-oxide sol, the flocculating power of the ions is in the order: | [Fe(CN)6]4- > PO43- > SO42- > Cl- |
| 42 | Gold sol is not a : | Macromolecular colloid |
| 43 | The incorrect statement about Hall-Héroult process is : | Oxidation state of oxygen changes in the overall cell reaction. |
| 44 | Select the correct statement: | Calcination of calcium carbonate is endothermic. |
| 45 | NO2 gas is : | Brown, acidic |
| 46 | Identify the incorrect statement from the following: | Ozone absorbs infrared radiation. |
| 47 | The correct decreasing order of boiling point of hydrogen halides is: | HF > HI > HBr > HCI |
| 48 | The synthetically produced radioactive noble gas by the collision of 24998Cf with 4820Ca is: | Oganesson |
| 49 | The transition element (≈ 5%) present with lanthanoid metal in Misch metal is : | Fe |
| 50 | Match the following: I. Zn2+ i. d8 conifugration II. Cu2+ ii. colourless III. Ni2+ iii. μ = 1.73 BM | I - ii, II - iii, III - i |
| 51 | Which of the following statements related to lanthanoids is incorrect? | Colour of Lanthanoid ion in solution is due to d- d transition |
| 52 | On treating 100 mL of 0.1 M aqueous solution of the complex CrCl3.6H2O with excess of AgNO3, 2.86 g of AgCl was obtained. The complex is: | (Cr(H2O)5Cl)Cl2.H2O |
| 53 | The complex compounds [Co(NH3)5SO4] Br and [Co(NH3)5Br]SO4 are : | Ionisation isomers |
| 54 | Which of the following statements are true about [CoF6]3- ion? I. The complex has octahedral geometry. II. Coordination number of Co is 3 and oxidation state is + 6. III. The complex is sp3d2 hybridised IV. It is a high spin complex. | I, III, and IV |
| 55 | A haloalkane undergoes SN2 or SN1 reaction depending on : | Solvent used in the reaction |
| 56 | 2-Methyl propane can be prepared by Wurtz reaction. The haloalkanes taken along with metallic sodium and dry ether are : | chloromethane and 2-chloropropane |
| 57 | In the following scheme of reaction,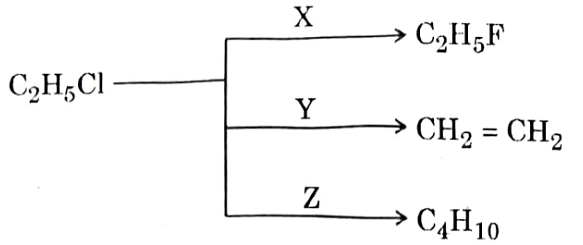 X, Y and Z respectively are: | Hg2F2 alcoholic KOH and Na in dry ether |
| 58 | 8:8 g of monohydric alcohol added to ethyl magnesium iodide in ether liberates 2240 cm3 of ethane at STP. This monohydric alcohol when oxidised using pyridinium-chlorochromate, forms a carbonyl compound that answers silver mirror test (Tollens' test,). The monohydric alcohol is : | 2,2-dimethyl propan-1-ol |
| 59 | When a tertiary alcohol 'A' (C4H10) reacts with 20% H3PO4 at 358 K, it gives a compound 'B (C4H8) as a major product. The IUPAC name of the compound 'B' is: | 2-Methylpropene |
| 60 | PCC is : | A complex of chromium trioxide with pyridine + HCI |
Download the question paper here:
KCET Chemistry Set B1 Question Paper 2024 (Shared by Samrudh Basapur)
KCET Chemistry Set D3 Question Paper 2024 (Shared by Tariq Aziz)
KCET Marks vs Rank Analysis with 2nd PUC Weightage
KCET Course-Wise Expected Cutoff 2024
| Name of the Course | Cutoff Link |
|---|---|
| B.Sc Agriculture | Expected KCET Agriculture Cutoff 2024 |
| Pharmacy | Previous Year's KCET Pharmacy Cutoff |
| B.Tech | Expected KCET B.Tech Cutoff 2024 |
KCET Subject-Wise Answer Key and Analysis 2024
Also Read |
| Links |
|---|
| KCET Result Expected Release Date 2024 |
| Previous Year's NIT Karnataka B.Tech CSE JEE Main Cutoff Ranks |
| Previous Year's IIIT Dharwad B.Tech CSE JEE Main Cutoff Ranks |


 Follow us
Follow us














