3 April Chemistry JEE Main Question Paper 2025 with Answer Key Session 2 (Image Credit: iStock)
3 April Chemistry JEE Main Question Paper 2025 with Answer Key Session 2 (Image Credit: iStock)3 April Chemistry JEE Main Question Paper 2025 : Students can access the JEE Main Chemistry question paper and unofficial answer key solutions for April 3, 2025, covering both Shift 1 and Shift 2. This resource features 25 memory-based chemistry questions, along with unofficial solutions, to help students gauge their performance. As the exam is held in online mode (CBT), these memory-based questions are a valuable study aid. We will provide downloadable PDFs, curated by top coaching institutes like Resonance, Vedantu, and Aakash, offering their respective answer keys in a clear and easily accessible format.
| JEE Main Question Paper 3 April 2025 Live Updates | JEE Main Expected Percentile Score April 2025 |
|---|
3 April Chemistry Question Paper 2025 Shift 2 with Answer Key
Obtain the Chemistry question paper and answer key solutions for the evening shift exam on Day 2 for Session 2 and calculate your scores.
Q.No. | Question | Answers |
|---|---|---|
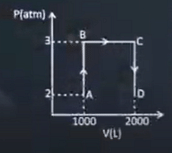
1 |
Find out magnitude of work done in the process ABCD (in kJ) (1 atm. Lit = 101.3 J)
| 304 |
| 2 | Amount of magnesium (Mg) (in mg) required to liberate 224 mL of H₂ gas at STP, when reacted with HCl. | 240 |
| 3 | Among Sc, Ti, Mn and Co, Calculate the spin only magnetic moment in oxidation state of metal having highest heat of atomisation. | 3 |
| 4 |  |  |
| 5 |
The correct IUPAC Name of the given compound is:
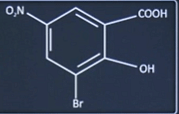 | 3-Bromo-2-Hydroxy-5-nitrobenzoic acid |
| 6 | At 715 mm pressure, 300 K, volume of N₂ (g) evolved was 80 mL by a 0.4 g sample of organic compound. Find % of N in organic compound 4. tension at 300 K = 15 mm | 20.95 |
| 7 | Which of the following reagent is used to prepare tribromoamiline ? | 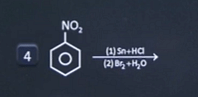 |
| 8 | 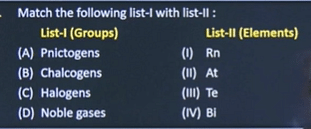 | A IV B III C III D I |
| 9 | Find orbital angular momentum for 2s and 2p energy levels |  |
| 10 |
Which of the following order is correct?
(A) Electronegativity : B > TI > In > Ga > Al (B) lonisation energy : B > TI > Ga > Al > In (C) Density: TI > In > Ga > Al > B (D) Size: B > Al > Ga > In > Al | (A, B, C) only |
| 11 |  | Br 2 /H 2 O |
| 12 |
Which of the following vitamins are fat-soluble?
B 12 , C, D, B, E | D and E |
| 13 |
Statement-I : CrO
3
is a strong oxidising agent
Statement-II: Cr +6 is more stable than Mo +6 considering the above statements, choose the correct option. | Both Statement I is correct but Stataemnt II is incorrect |
| 14 |
Which of the following compound or complex ions is/are diamagnetic in nature?
(a) CrO 3 (b) [Fe(CN) 6 ] 4- (c) [Co(H₂O 3 ),F 3 ) (d) [Cr(NH 3 ) 6 ] 3+ | a, b and c only |
| 15 | 20 mL 1M NaOH is mixed with 10 mL 2M HCI which is further diluted to 100 mL. Find concentration of final solution? | 0.2 M |
| 16 | Which of the following statement is correct w.r.t. Arrhenius equation? | Dimension of K and A are same |
| 17 |  | P (4) Q (3) R (2) S (1) |
| 18 |
Statement-I: Wet cotton clothes made up of cellulose based carbohydrates take a comparatively longer time to get dried than wet Nylon based clothes.
Statement-II: Both form intermolecular H-bonter molecules | Both statement-I and statement-ll are correct |
| 19 | C 9 H 12 is a derivative of benzene, how many total structural isomers of the compounds are possible. | 4 |
| 20 | 'x' g of nitrobenzene gives 4.2 g 1, 3-dinitrobenzene with 100% yield. Find the value of | 3.075 |
3 April Chemistry Question Paper 2025 Shift 1 with Answer Key
For the morning shift of the second session exam on Day 2, access the Chemistry question paper along with the answer key solutions below.
Q.No. | Question | Answers |
|---|---|---|
1 | Which of the following ion shows spin only magnetic moment of 4.9 B.M. | Cr 2+ |
| 2 | Which of the following has the highest atomic number? | Po |
| 3 | 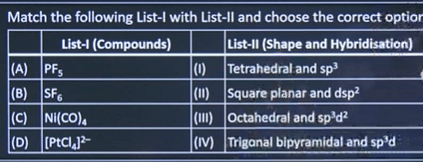 | A-IV B-III C-I D-II |
| 4 | 2 moles each of ethylene glycol and glucose are mixed with 500 g of water. Find the boiling point of solution. Given: K = 0.52 K kg mol-1 | 377.17K |
| 5 | 0.5 g of an organic compound gives 1.46 g CO₂ and 0.9 g H₂O. What is the % of carbon in reanic sample. | 80 |
| 6 | Which of the following is more acidic than others? |  |
| 7 | Which of the following will give 3-methyl-6-oxoheptanal as the product of reductive ozonolysis? | 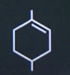 |
| 8 |
Observe the following reaction sequence
(A) + (NaNO 2 + HCI 0-5°C Which of the following options has the correct structure (A) and (B) respectively. | 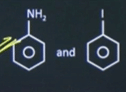 |
| 9 |
Q. The given reaction is at equilibrium starting with only PCl5
PCl 5 (g) PCl 3 (g) + Cl 2 (g), when addition of Xe gas takes place at constant pressure, then which following is correct? | PCl 3 and Cl₂ will have same concentration at new equilibrium. |
| 10 |
Consider the following statements
Statement I: N-N has less bond strength than P-P Statement II: All group-15 elements in +3 oxidation state undergo disproportionation In the light of above statements, choose the correct option. | Statement I is correct and Statement II is incorrect |
| 11 | Which of the following property shows irregular trend in group 16? | Electron affinity |
| 12 |
Which of the following statement(s) is/are incorrect
I. NO₂ dimerises easily II. NF 5 does not exist but PF5 exists III. The oxides N 2 O 5 and P 2 O 5 are purely acidic but As 2 O 5 and Sn 2 O 5 are basic IV. Nitrogen cannot form dπ-ρπ bond as the heavier elements can | Only III |
| 13 |
Consider the following complex ions
(a) [Co(NH 3 ) 6 ] 3+ (b) [Co(NH 3 )Cl] 2+ (c) [Co(NH 3 ) 5 (H 2 O)] 3+ (d) [Co(CN) 6 ] 3- Choose the correct order of wavelength absorbed by complex ions | b>c>a> d |
| 14 |
Q. Arrange the following metal ions in the decreasing order of their molar conductivity in aqueous solution.
Ca 2+ , Mg 2+ Na + , K + |
Ca
2+
> Mg
2+
> K
+
> Na
+
|
| 15 | Which of the following represents L-form of fructose? | 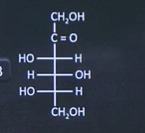 |
| 16 |  | (a) and (d) |
| 17 | Correct set of four Quantum numbers for last electron of Cr 3+ ion is | n = 3,1 = 2, m = 0, s = +1/2 |
| 18 |
Given below are two statements about X-ray spectra of elements:
Statement (I): A plot of √v (v = frequency of X-rays emitted) vs atomic mass is a straight line Statement (II): A plot of v (v = frequency of X-rays emitted) vs atomic number is a straight line. In the light of above statements, choose the correct answer from the options given | Both Statements I and II are incorrect |
| 19 | In two first order reactions initial concentration of [A] = 8[B]. Find the time after which concentration of A and B become equal. Given that (t1/2) = 20 min and (11/2)8 = 80 min. | 80 |
| 20 |
How many of the following statements are correct?
(a) First ionisation energy of Boron is more than Beryllium. (b) Lithium is strong reducing agent (c) Electronegativity of carbon is 2.5 (approx.) in CCl4 (d) Removal of electron from isolated gaseous atom is endothermic and addition of electron to isolated gaseous atom is generally exothermic | (b) (c) and (d) |
| 21 |
0.42 g of the following compound (X) is subjected to analysis for estimation of volume of N₂ gas by Duma's method
What is the volume of N₂ gas evolved in mL at STP (1 atm pressure and 273 K temperature) to the nearest integer. 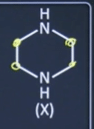 | 86 |
Also read | 2 April 2025 JEE Main Chemistry Question Paper with Answer Key
Download the JEE Main Chemistry question paper and answer key for April 3, 2025, from CollegeDekho, as provided above in PDF format:
Shift | Chemistry Question Paper with Answers Links |
|---|---|
Shift 1 | |
Shift 2 |
3 April 2025 JEE Main Question Paper: Physics and Mathematics
Check and download the JEE Main question paper for Physics and Mathematics from April 3, 2025, here.
Parameter | Question Paper and Answer Key Link |
|---|---|
Physics | 3 April JEE Main Physics Question Paper 2025 with Answer Key |
Mathematics | 3 April Mathematics JEE Main Question Paper 2025 with Answer Key |
3 April Chemistry JEE Main Question Paper 2025 PDFs by Coaching Institutions
The Chemistry answer keys and question paper PDF links for both Shift 1 and Shift 2 of the JEE Main exam on April 3, 2025, will be made available, as and when published by Resonance, Aakash, and Vedantu in the table below.
Coaching Institute | Shifts | 3 April Physics Question Paper with Answer Key PDFs |
|---|---|---|
Vedantu | Shift 1 | Vedantu 3 April Chemistry Shift 1 JEE Main Question Paper 2025 |
Shift 2 | Vedantu 3 April Chemistry Shift 2 JEE Main Question Paper 2025 | |
Aakash | Shift 1 | Aakash 3 April Chemistry Shift 1 JEE Main Question Paper 2025 |
Shift 2 | Aakash 3 April Chemistry Shift 2 JEE Main Question Paper 2025 | |
Resonance | Shift 1 | Resonance 3 April Chemistry Shift 1 JEE Main Question Paper 2025 |
Shift 2 | Resonance 3 April Chemistry Shift 2 JEE Main Question Paper 2025 |
Marks vs Percentile vs Rank Analysis |
| Marks Range | Expected Percentile and Rank Analysis Link |
|---|---|
| 50 Marks | 50 Marks in JEE Mains Percentile and Rank 2025 |
| 60 Marks | 60 Marks in JEE Mains Percentile and Rank 2025 |
| 70 Marks | 70 Marks in JEE Mains Percentile and Rank 2025 |
| 80 Marks | 80 Marks in JEE Mains Percentile and Rank 2025 |
| 90 Marks | 90 Marks in JEE Mains Rank and Percentile 2025 |
Detailed Percentile vs Marks vs Rank |
| Percentile | Marks vs Rank Link |
|---|---|
| Day-wise marks vs percentile | JEE Main 2025 Session 2 Day-wise Expected Marks vs Percentile |
| 99.9 | 99.9 Percentile in JEE Main 2025 Marks and Rank |
| 99.5 | 99.5 Percentile in JEE Main 2025 Marks and Rank |
| 99 | 99 Percentile in JEE Main 2025 Marks and Rank |
| 98 | 98 Percentile in JEE Main 2025 Marks and Rank |
| 97 | 97 Percentile in JEE Main 2025 Marks and Rank |
| Also Check | | JEE Main Expected Percentile Rank April 2025 |
Important Links |
| Concept | Link |
|---|---|
| Question Paper (All Shifts) | JEE Main 2025 Session 2 Question Paper with Answer Key |
| 3 April Shift 1 Paper Review | JEE Main 3 April Shift 1 Paper Review 2025 |
| 3 April Shift 2 Paper Review | JEE Main 2025 Shift 2 April 3 Paper Review |
| 3 April Marks vs Percentile | JEE Main 2025 April 3 Expected Marks vs Percentile |
| Expected Cutoff | JEE Main Expected Cutoff Percentile 2025 |
| General Category NIT Cutoff Rank | Minimum Rank required for General Category in JEE Main 2025 for NIT admission |
Subject-Wise Expected Percentile Score 2025 |
| Subject Name | Link |
|---|---|
| Physics | JEE Main Physics Expected Percentile Score 2025 |
Keep visiting CollegeDekho for the latest Education News on entrance exams, board exams and admissions. You can also write to us at our email ID news@collegedekho.com.
Are you feeling lost and unsure about what career path to take after completing 12th standard?
Say goodbye to confusion and hello to a bright future!

Was this article helpful?





 Follow us
Follow us