 WBJEE Chemistry Answer Key 2025 Unofficial for all Sets
WBJEE Chemistry Answer Key 2025 Unofficial for all Sets
WBJEE Chemistry Answer Key 2025 Unofficial
: The West Bengal Joint Entrance Examinations Board (WBJEEB) conducts the WBJEE 2025 exam today, April 27, 2025. As per the pattern, the paper includes three sections: Mathematics, Physics, and Chemistry. Candidates who have appeared for the exams shall access WBJEE Chemistry Answer Key 2025 Unofficial here, prepared by subject experts. Candidates will be able to check the correct answers for all 40 questions from the Chemistry section here for all sets. This is an unofficial answer key; the official answer key will be released by WBJEEB through the official website, based on which the results will be declared. The unofficial WBJEE Chemistry Answer Key 2025 will guide the candidates to self-analyse their performances and estimate the expected scores.
Also Read |
WBJEE Unofficial Answer Key 2025 LIVE Updates
WBJEE Chemistry Answer Key 2025 Unofficial
The unofficial answer key for WBJEE 2025 will be provided below in a set-wise PDF format for students to download and verify their answers.
| Sr.No. (Not Q.No.) | Question | Answer |
|---|---|---|
| 1 | Increasing order of solubility of AgCl in (I) H 2 O (ii) IMNaCl (aq.), (iii) IMCaCl 2 (aq.) and (iv) IM NaNO 3 (aq.) solution | CaCl 2 < NaCl < H 2 0 < NaNO 3 |
| 2 | The bond order of HeH + is | 1 |
| 3 | If the three elements A, B, C crystalise in a cubic solid lattice with B atoms at the cubic centres, C atoms at the centre of edges and A atoms at the corners, then formula of the compound is | ABC 3 |
| 4 | An LPG cylinder weighs 15.0 kg when empty. When full, it weighs 30.0 kg and shows a pressure of 3.0 atm. In the course of usage at 27°C, the mass of the full cylinder is reduced to 24.2kg. The volume of the used gas in cubic metres at the normal usage condition (1 atm and 27°C) is (assume LPG to be normal butane and it behaves ideally). | 2.46 m 3 |
| 5 | P and Q combines to form two compounds PQ 2 and PQ 3 . If 1 g PQ 2 is dissolved in 51 g benzene the depression of freezing point becomes 0.8°C. On the other hand if 1 g PQ 3 is dissolved in 51 g of benzene, the depression of freezing point becomes 0.625°C. The atomic mass of P and Q are (K of benzene=5.1 K kg mol -1 ) | 55,45 |
| 6 | For a chemical reaction, half-life period (f1/2) is 10 minutes. How much reactant will be left after 20 minutes if one starts with 100 moles of reactant and the order of reactions be (I) zero, (ii) one and (iii) two? | 0,25,33.33 |
| 7 | Which one among the following compounds will most readily be dehydrated under acidic condition? | 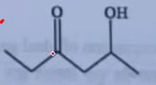 |
| 8 | Kieldahl's method cannot be used for the estimation of nitrogen in which compound? | C 6 H 5 -N=N-C 6 H 5 |
| 9 | Which of the following compounds is most reactive in S N 1 reaction? | 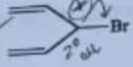 |
| 10 | Equal volumes of two solutions A and B of a strong acid having pH=6.0 and pH+4.0 respectively, are mixed together to form a new solution. The pH of the new solution will be in the range | between 4 and 5 |
| 11 | Adiabatic free expansion of ideal gas must be | Isothermal |
| 12 |
5
B
10
+
2
He
4
➞ X +
0
n
2
In the above nuclear reaction "X" will be | 7 N 13 |
| 13 |
In the following reaction, the major product (H) is
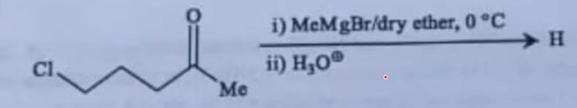 | 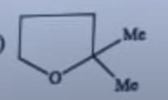 |
| 14 | Which of the following hydrogen bonds is likely to be the weakest? | C--H ...O |
| 15 | How many oxygen atoms are present in 0.36 g of a drop of water at STP? | 1.205 x 10 22 |
| 16 | Which of the following oxides is paramagnetic? | NO 2 |
| 17 | 360 cm 3 of a hydrogen diffuses in 30 minutes, while under the same conditions 360 cm 3 of SO 2 gas diffuses in one hour. The molecular formula of the hydrocarbon is | C 2 H 6 |
| 18 | The molar conductances of Ba(OH) 2 , BaCl 2 and NH 4 Cl at infinite dilution are 523.28, 280.0 and 129.8 S cm 2 mol -1 respectively. The molar conductance of NH 4 OH at infinite dilution will be | 251.44 S cm 2 mol -1 |
| 19 | What is the four electrons reduced from O 2 ? | Oxide |
| 20 | How many electrons are needed to reduce N 2 to NH 3 ? | 6 |
| 21 | An optically active alkene having molecular formula C 3 H 16 gives acetone as one of the products on ozonolysis. The structure of the alekene is | 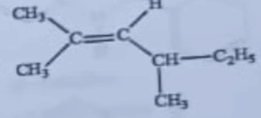 |
| 22 |
increasing order of the nucleophilic substitution of following compounds is
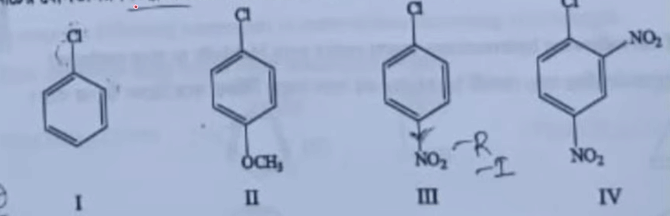 | II < I < III < IV |
| 23 |
Identify the major product (G) in the following reaction
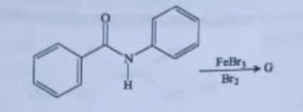 | 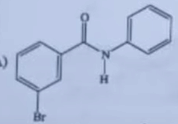 |
| 24 | Which of the following hydrocarbons reacts easily with MeMgBr to give methane? |  |
| 25 |
The major product (F) in the following reaction is
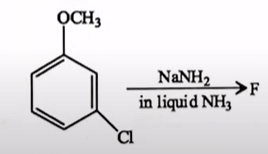 | m-Anisidine |
| 26 | The common stable oxidation states of Eu and Gd are respectively | +3 and +3 |
| 27 |
Arrange the following compounds in order of their increasing acid strength
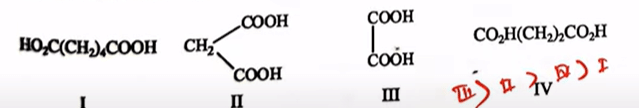 | I < IV < II < III |
| 28 | The number of Ione pair of electrons and the hybridization of Xenon (Xe) in XeOF 2 are | 2, sp 3 d |
| 29 | The coagulating power of electrolytes having ions Na + , Al 3+ and Ba 2+ for As 2 S 3 sol increases in the order | Na + < Ba 2+ < Al 3+ |
| 30 | The number of terminal and bridging hydrogens in B 2 H 6 are respectively | 4 and 2 |
| 31 | Consider the following gas phase dissociation, PCl 5 (g) ⇌ PCl 3 (g) + Cl 2 (g) with equilibrium constant K P at a particular temperature and at pressure P. The degree of dissociation (α) for PCl 5 (g) is | 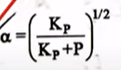 |
| 32 | Compound given below will produce effervescence when mixed with aqueous sodium bicarbonate solution | 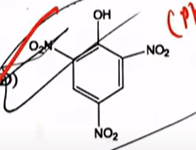 |
| 33 | As per the following equation, 0.217 g of HgO (molecular mass= 217 g mol -1 ) reacts with excess iodide. On titration of the resulting solution, how many mL of 0.01 M HCl is required to reach the equivalence point? | 200 mL |
| 34 | An egg takes 4.0 minutes to boil at sea level where the boiling point of water is T 1 K, where as it takes 8.0 minutes to boil on a mountain top where the boiling point of water is T 2 K. The activation energy for the reaction that takes place during the boiling of egg is |  |
| 35 | 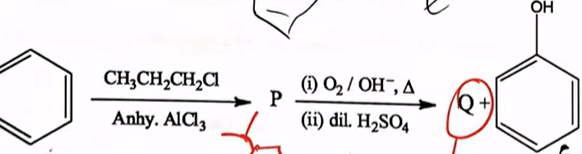 The major product "P" and "Q" in the above reactions are | 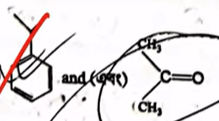 |
| 36 | Which pair of ions among the following can be separated by precipitation method? | All options |
| 37 |
Identify "P" and "Q" in the following reactions
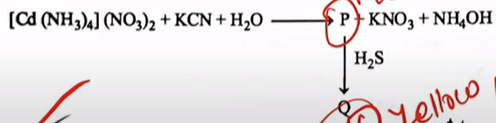 | P = K 2 [Cd (CN) 4 ], Q = CdS |
| 38 | X is an extensive property and x is an intensive property of a thermodynamic system. Which of the following statement(s) is (are) correct? | xX is extensive, X/x is extensive |
| 39 |
Which of the following statement(s) is/are correct about the given compound?
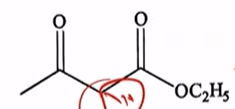 | All options |
| 40 | The compound(s) showing optical activity is/are: | 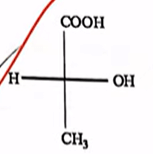 and 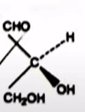 |
Also Read | WBJEE Result Expected Release Date 2025
As per the question paper pattern, the Chemistry section will be divided into three categories: Category 1 with 30 questions, and Categories 2 and 3 with 5 questions each. The marking scheme allots 1 mark for each correct answer for Category 1 and 2 marks for each correct answer for Category 2 and 3. Further, the negative marking for Category 1 is 1/4th mark, for Category 2, ½ mark will be deducted for an incorrect answer. Category 3 has no negative marking.
WBJEE Subject-wise Answer Key 2025 Unofficial |
Subjects | PDF Download Links |
|---|---|
Mathematics | |
Physics |
WBJEE 2025 Expected Rank |
| Particulars | Links |
|---|---|
| Overall | WBJEE Expected Rank 2025 GMR and PMR |
| 100 Marks | WBJEE 100 Marks vs Expected Rank 2025 |
| 50 Marks | 50 Marks in WBJEE 2025 Expected Rank |
| 20 Marks | 20 Marks in WBJEE 2025 Expected Rank |
Keep visiting CollegeDekho for the latest Education News on entrance exams, board exams and admissions. You can also write to us at our email ID news@collegedekho.com.
Are you feeling lost and unsure about what career path to take after completing 12th standard?
Say goodbye to confusion and hello to a bright future!

Was this article helpful?





 Follow us
Follow us












