Updated By Jahanvi Bakshi on 20 Aug, 2025 22:19
The GBPUAT syllabus 2026 for UG, PG, and PhD courses covers subjects like Physics, Chemistry, Biology, Mathematics, Agriculture, and more. Get the direct link to download the syllabus for GBPUAT 2026 here!
Your Ultimate Exam Preparation Guide Awaits!
The GBPUAT Syllabus 2026 will be released by the Govind Ballabh Pant University of Agriculture and Technology, Uttarakhand, after the GBPUAT 2026 admission prospectus and notification are released on the official website @gbpuat.ac.in.
The syllabus for GBPUAT 2026 examination will cover important topics from Physics, Chemistry, Biology, Mathematics, Agriculture, and other disciplines. The undergraduate syllabus is different from the GBPUAT PG syllabus. The GBPUAT UG syllabus covers Mathematics, Botany, Zoology, Chemistry, and Agriculture. Whereas, the PG syllabus for GBPUAT 2026 will consist of Biological Sciences, Physical Sciences, and MCA.
Students must go through the syllabus and GBPUAT 2026 exam pattern as part of their preparation to ace this university-level entrance examination. With a clear understanding of the Pantnagar University entrance exam syllabus and important topics, students will be able to perform better in the GBPUAT 2026 entrance exam.
Students can download the undergraduate and master's programme syllabus from the table below.
| Programme | PDF Download |
|---|---|
| Undergraduate Programme | Click Here |
| Masters Programme | Click Here |
The revised syllabus for GBPUAT 2026 entrance examination for undergraduate courses has been provided in detail below:
The updated syllabus for GBPUAT Mathematics section is provided in detail below:
| Probability | Linear Programming |
|---|---|
| Three - dimensional Geometry | Vectors |
| Differential Equations | Applications of the Integrals |
| Integrals | Applications of Derivatives |
| Continuity and Differentiability | Determinants |
| Matrices | Inverse Trigonometric Functions |
| Relations and Functions | -- |


Check the GBPUAT syllabus 2026 for Physics below:
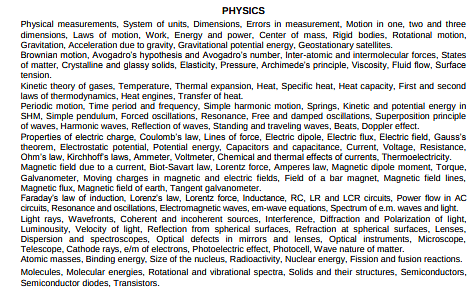
Check GBPUAT 2026 syllabus for Botany & Zoology below:
| Plant tissue culture, biotechnology and its applications | Natural resources and their conservation |
|---|---|
| Classification of the plant kingdom | Plant taxonomy and elementary study |
| Elementary study of Plant Physiology | Cell and cellular functions |
| Development of male and female gametophytes, pollination, fertilization and development of the embryo | Germination, growth and development |
| Flower, inflorescence, seed and fruits | Morphology of root, stem and leaf and their modifications |

Check the GBPUAT syllabus 2026 for Chemistry below:
| Lipids | Nucleic Acids |
|---|---|
| Proteins & Enzymes | Amino Acids and Peptides |
| Carbohydrates | Bio-Chemistry |
| Synthetics & Natural Polymers | Organic Chemistry Based on Functional Groups I and II |
| Halides & Hydroxy Compounds | Purification and Characterisation of Organic Compounds |
| Preparation and Properties of Hydrocarbons | Hydrocarbons |
| Carbon and It's Compound Introduction to Carbon Compounds | Surface and Catalysts |
| Colloids and Macromolecules | Solutions |
| Nuclear and Radiochemistry | States of Matter |
| Chemical Kinetics | Redox Reactions |
| Ionic Equilibrium in Solutions | Chemical Equilibrium and Phase Equilibrium |
| Chemical Thermodynamics | Coordination Chemistry and Organometallics |
| Chemistry of Representative Elements | Chemical Bonding and Molecular Structure |
| Periodic Properties of Elements | Atoms and Atomic Structure |



Check the GBPUAT 2026 syllabus for Agriculture below:
| Animal Husbandry and Veterinary Science | Agricultural Economics |
|---|---|
| Agricultural Engineering | Horticulture |
| Agronomy | --- |

Students must go through the detailed exam pattern and syllabus before they appear for the GBPUAT exam 2026. This section contains the syllabus for GBPUAT PG courses 2026:
Students can refer to the table attached below to check out the list of important topics covered under the Life Sciences subject in the GBPUAT (PG) postgraduate syllabus:
Unit | Topics |
|---|---|
1 | Morphology and anatomy of land plants, Cell cycle, cell division, senescence, the life cycle of an angiosperm, pollination, fertilization, embryogenesis, seed formation, seed storage proteins, seed dormancy and germination. Concept of cellular totipotency, organogenesis and somatic embryogenesis, Principles of Mendelian inheritance, linkage, recombination and genetic mapping; extrachromosomal inheritance; gene mutation, chromosome aberrations (numerical and structural), transposons. Introduction to Plant Breeding. Pteridophytes and Bryophytes, Taxonomy and. Plant Geography |
2 | Diversity, distribution, systematics and phylogeny of animals, Origin of life, history of life on earth, evolutionary theories, natural selection, adaptation, speciation. Principles of inheritance, the molecular basis of heredity, the genetic material, transmission of genetic material, Structure of cell, cellular organelles and their structure and function, cell cycle, cell division, Comparative physiology of different systems, Parasitic organisms and host-parasite relationship, Immune response, cellular and humoral immunity, the evolution of the immune system: Embryonic development, cellular differentiation, organogenesis. metamorphosis, the genetic basis of development. The ecosystem, habitats, species diversity, zoogeography and Animal behaviour |
3 | Structure of atoms, molecules and chemical bonds, Isomerism, hydrogen bond and hydrophobic interaction in biomolecules; Chemistry of biomolecules-carbohydrates, amino acids, proteins, lipids and nucleic acids, Enzymes and their kinetics, factors affecting enzyme activity, Competitive and non-competitive inhibitions. Coenzymes and cofactors, metabolism of carbohydrates, fatty acids and proteins. eukaryotic genome organization (chromatin structure), Genetic code, replication, transcription and translation. Regulation of gene expression, Plant pigments, Secondary metabolites, Vitamins, Hormones and metabolic regulation |
4 | Spontaneous generation theory-Grem theory-Discovery of antibiotics-Types of microscopes-Principles and equipment of different kinds of sterilisation-staining Techniques-Nutritional types of bacteria-Growth curve factors influencing bacterial growth-Fermentation and other Metabolic pathways: Principle and ApplicationClassification of Bacteria-Gene transfer methods in microorganisms Antigen and antibody reaction. Role of microbes in carbon and nitrogen cycles-Influence of Rhizosphere on soil microorganism. Microbes in Industry and health |
5 | Plant physiology and its significance in agriculture; physical properties and chemical constitution of protoplasm; plant cell water relation-imbibition, surface tension, diffusion, osmosis; absorption and translocation of water and nutrients; transpiration, guttation, mineral deficiencies and their symptoms; physiological: disorders, correction, hydroponics, foliar nutrition aerobic and anaerobic respiration; Photorespiration Factors affecting respiration and Photorespiration. Photosynthesis- modern concept and the factors affecting photosynthesis, nitrogen fixation growth development and differentiation; growth hormones, growth retardants, growth inhibitors and their use in agriculture; tropism in plants photoperiodism and vernalization; seed dormancy, germination; fruit ripening process and its control |
6 | Concepts and scope of biotechnology, Tissue culture and its applications, Micropropagation, Meristem culture and production of virus-free plants. Anther and microspore culture. Embryo and ovary culture. Protoplast isolation, fusion, and somatic hybridization, cybrids, some clones, synthetic seeds, In vitro germ conservation, Cryopreservation, Organellar DNA, satellite and repetitive DNA, DNA repair, Recombinant DNA technology, Cloning vector, Restriction enzymes, Gene cloning. Methods of gene transfer in plants, Achievements and recent developments of genetic engineering in agriculture. |
7 | Ecology and its relevance to life, natural resources-their management and conservation, Climatic elements as factors of crop growth, Impact of change in environment on cropping patterns, Change in the environment due to agricultural environmental pollution and associated hazards to crops and animals, Human liquid and solid waste disposal, pollution prevention and remediation, Concepts and dynamics of ecosystem component, Food chain and energy flow, Productivity and biogeochemical cycle, Types of the ecosystem, Population ecology and biological control, Community structure and organization, Sustainable development, Economic importance of microbes, plants and animals |
8 | Electronic configuration of elements, periodic classification of elements, atomic number, atomic and ionic radii, ionization potential, electron affinity and electronegativity, the electronic theory of valency, sigma and pi bonds, hybridization and directional nature of covalent bonds, metallic bonds, VSEPR theory, V.B. and MO theory, ionic solids and weak interactions, Lewis and Bronsted theories of acids and bases, hard-soft acid and bases (HSAB), oxidation states and oxidation number, common oxidizing and reducing agents, Ionic equations. Natural and artificial radioactivity, radioactive decay, nuclear fission and fusion. Chemistry of the common elements and their compounds. Principles of extraction isolation (and metallurgy) of important elements. Chemistry of transitional elements, lanthanides and actinides. Structures of hydrogen peroxide, diborane, aluminium chloride and the important oxyacids of nitrogen, phosphorus, chlorine and sulphur. Interhalogen compounds. Outlines of the manufactures of sodium carbonate, sodium hydroxide, ammonia, nitric acid, sulphuric acid, cement, glass, ceramics and artificial fertilizers. Inert gases: Isolation and Chemistry, the structure of inert gas compounds. Werner‟s theory of coordination compounds, V.B. and M.O. theory of bonding in metal complexes, electronic spectrum, magnetic and spectral properties of metal complexes. Organometallic compounds. Bioinorganic chemistry, the biological role of alkaline earth metal ions, metalloporphyrins. Analytical Chemistry: Principles and methods of chemical analysis, principles involved in separation techniques, chromatography |
9 | Modern concepts of covalent bonding, bond lengths, energy and bond angles, electron displacements, inductive, electromeric, mesomeric and hyper conjugative effects, resonance ad its applications to organic chemistry, tautomerism, effects of structure on chemical reactions, dissociation constants. Mechanism of organic reactions: Types of reagents and organic reactions, reaction intermediates, product analysis, isotope effects, kinetic and stereochemical studies. Stereochemistry: Optical and geometrical isomerism, chirality, enantiomers, stereogenic centres, diastereomers, resolution and racemization, relative and absolute configuration, sequence rules, E&Z and R&S nomenclature, the concept of conformation and conformational analysis of ethane, butane and cyclohexane and sugars. Chemistry and reactions of derivatives of aliphatic and aromatic compounds including Alkanes, alkynes and alkenes. arenes and aromaticity, benzene and polynuclear hydrocarbons, alkyl and aryl halides, SN1, SN2 and SNi reactions, nuclear and side-chain reactions, aromatic substitutions reactions, elimination reactions. Aliphatic and aromatic alcohols and phenols, ethers and epoxides, aliphatic and aromatic aldehydes and ketones, aliphatic and aromatic carboxylic acids and their derivatives, aliphatic and aromatic amines and amides, synthetic applications of diazonium salts. Amino-acids. Reactions and applications of organometallic compounds, Acetoacetic and malonic esters, Organic synthesis via enolates. Heterocyclic compounds, pyridine, quinoline, thiophene, furan and pyrrole. Important organic name reactions and rearrangements of synthetic importance. Carbohydrates, classification and general reactions, glucose, fructose and amino acids and proteins, terpenoids and alkaloids. Polymers, dyes and pigment. Theory and application of spectral techniques, UV, IR and NMR in structure elucidation of simple organic molecules |
10 | Elementary quantum mechanics Gaseous states: Kinetic theory of gases and gas laws, Maxwell‟s law of distribution of velocities, Van der Waal‟s equation, Law of corresponding states, Liquification of gases, Ratio of Cp/CV. Thermodynamics: The first law of thermodynamics, Isothermal and adiabatic expansion, Enthalpy, heat capacities, Thermochemistry –heats of reaction, formation, solution and combustion, Calculation of bond 53 energies, Kirchhoff equation, Criteria for spontaneous changes, the second law of thermodynamics entropy. Free energy, criteria of thermodynamic equilibrium. Solutions: osmotic pressure lowering of vapour pressure, depression of freezing point, the elevation of boiling point, determination of molecular weights, association and dissociation of solutes. Chemical equilibrium, the law of mass action and its applications to homogeneous and heterogeneous equilibrium, Le Chatelier‟s principle, Influence of temperature on chemical equilibrium. Electrochemistry: Faraday‟s laws of electrolysis, the conductivity of an electrolyte: equivalent conductivity and its variation with dilution, the solubility of sparingly soluble salts, electrolytic dissociation, Ostwald‟s dilution law, the anomaly of strong electrolytes, solubility product, the strength of acids and bases: hydrolysis of salts, hydrogen ion concentration buffer action, the theory of indicators. Electrochemical cells: Reversible cells, standard hydrogen and calomel electrodes and redox-potentials, concentration cells, determination of pH transport number and ionic product of water, Potentiometer titration, chemical kinetics: Molecularity and order of a reaction, First-order and second-order reactions, Determination of order of a reaction, temperature coefficients and energy of activation, Collision theory of reaction rates, Activated complex theory. Phase rule: Explanation of the terms involved, Applications to one and two-component system, reduced phase rule, distribution law. Colloids: General nature of colloidal solutions and their classification, general methods of preparation and properties of colloids, coagulation, protective action, gold number, adsorption phenomenon and adsorption isotherms. Catalysis: Homogeneous and heterogeneous catalysis, catalytic promoters and poisons. |
The following table consists GBPUAT PG Physical Sciences Syllabus 2026:
Unit | Topics |
|---|---|
1 | Gradient, Divergence and Curl of Vector fields, Gauss‟s, Stoke‟s and Green‟s theorems. Newton‟s laws, Galilean invariance, Non-relativistic motion of charged particles in an electric and magnetic field, conservation of linear, angular momenta and energy, Collisions, Centre of mass frame. Inverse-square law force, Kepler‟s laws. Harmonic oscillator. Damped harmonic oscillator, Quality factor. Coulomb‟s law, field due to a charge distribution, Gauss‟s theorem and its applications, Line integral of the electrical field, electric potential, Force on a surface charge, Energy associated with the electric field. Current density, charging and discharging of a condenser through a resistance. Force on a moving charge, Fields due to a Helmholtz coil, Solenoid and a current loop, magnetic flux, Faraday‟s law in differential form, Self and mutual inductance, RL and RC circuits. Dielectrics, Moments of a charge distribution, Torque and force on a dipole in an electric field, Induced dipole moment, Polarisability, Qualitative idea about dia, para and Ferromagnetism, Magnetic susceptibility, Langevin‟s theory of paramagnetism, Hysteresis phenomenon, AC circuits |
2 | Rutherford‟s atomic model, Bohr‟s model and spectra of hydrogen atom fine structure, Sommerfield model, spatial quantization and electron spin. Normal Zeeman effect. X-ray spectra, Moseley‟s law, Luminescence, Principle and working of different kinds of Lasers, Raman effect. Laws of thermodynamics, Entropy, Cp and Cv of a gas. Macroscopic and microscopic systems, Internal and external energy states of a molecule, Reversible and irreversible processes, production of low temperatures. Maxwell‟s thermodynamic relationships, Triple point, applications of Maxwell‟s thermodynamical relations. Introduction to cryogenics and refrigeration. Black body radiation and different radiation laws. Einstein‟s theory of specific heat and its limitations, Lattice vibrations, phonons-Debye‟s theory of specific heat of solids, the specific heat of diatomic gases and its variation with temperature. Black body spectrum, photoelectric effect and Compton effect. De Broglie‟s waves, Group and phase velocities, Uncertainty principle. Schrödinger‟s equation, Operators, Expectation values. Applications of Schrödinger‟s equation and its various applications. Michelson Morley experiment, Postulates of special relativity, Lorentz transformations, Relativistic kinematics |
3 | Fermat‟s Principle, Cardinal points, telescopic combinations, Interference and diffraction of light, Rayleigh criterion, resolving power of telescope and microscope, Grating, Resolving power of a grating, Polarization and different kinds of polarized light, Double refraction, optical activity Electromagnetic theory. Kirchoff's Laws, Thevenin's & Nortons Theorems, Filters, VTVM, CRO. Semiconductor Devices, diodes and transistors, FET, MOSFETS, UJT, Thermistors, Rectifiers, Power supply. Different types transistor-based amplifiers and oscillators, Multivibrators, Logic gates and Boolean Algebra |
4 | Crystal structure, Unit cell, Bravis lattices, Miller indices, X-ray diffraction, Bragg‟s law. Lattice vibrations: Free electron theory of metals, Distinction between conductors, semiconductors and insulators, Intrinsic and Extrinsic semiconductors. Probability, Ensemble and average properties, Equilibrium and fluctuations, constraints, Equilibrium between two systems in thermal contact, the ß parameter, Entropy and probability Boltzmann entropy relation, Statistical interpretation of second law of thermodynamics. Maxwellian Distribution of Speeds in an ideal gas. Bose-Einstein and Fermi-Dirac Statistics, Free electrons in a metal, photons in black body chamber, Fermi level and Fermi energy. Structure of nucleus; Liquid drop model and semi-empirical mass formula, nuclear reactions, nuclear fission and fusion, elementary particles. Artificial nuclear transmutation, Particle accelerators & detectors, α, β, γ. decay |
5 | Real analysis: continuity and discontinuity, Reimann Integral and its applications. Algebra of integral function, convergence and divergence. Different methods in Hydrodynamics in the equation of continuity in different systems. Equation of motion and Bernoulli‟s equation with different cases. Motion in 2D and its applications. Differential and Integral calculus: successive differentiation, Different form of theorems, Tangents and normal, Maxima and Minima with different cases. Various types of integral form, Beta and gamma functions. Linear Algebra: Group, ring field, Integral domain and vector space with examples and theorems. Differential equations and Laplace transformation, vector analysis and Analytical Geometry, mechanics and complex analysis |
6 | General Statistics, Probability Distributions, Test of Significance, Analysis of Variance, Sampling Theory, Design of Experiment, Time-series Analysis, Index Numbers, Statistical Quality Control, Vital statistics, Statistical organizations in India |
7 | Introduction to computers, fundamentals, peripherals of PCs, software and Hardware. Evolution. Operating system. Structural computer Languages: programming in C, UNIX, WINDOWS Operating Systems. Number systems and computer architecture. Computational methods for Numerical Analysis: Algebraic and Transcendental equations, systems of simultaneous equations. Interpolation and Differentiation, solution of ordinary differential equations with initial value and boundary value problems |
8 | Electronic configuration of elements, periodic classification of elements, atomic number, atomic and ionic radii, ionization potential, electron affinity and electronegativity, the electronic theory of valency, sigma and pi bonds, hybridization and directional nature of covalent bonds, metallic bonds, VSEPR theory, V.B. and MO theory, ionic solids and weak interactions, Lewis and Bronsted theories of acids and bases, hard-soft acid and bases (HSAB), oxidation states and oxidation number, common oxidizing and reducing agents, Ionic equations. Natural and artificial radioactivity, radioactive decay, nuclear fission and fusion. Chemistry of the common elements and their compounds. Principles of extraction isolation (and metallurgy) of important elements. Chemistry of transitional elements, lanthanides and actinides. Structures of hydrogen peroxide, diborane, aluminium chloride and the important oxyacids of nitrogen, phosphorus, chlorine and sulphur. Interhalogen compounds. Outlines of the manufactures of sodium carbonate, sodium hydroxide, ammonia, nitric acid, sulphuric acid, cement, glass, ceramics and artificial fertilizers. Inert gases: Isolation and Chemistry, the structure of inert gas compounds. Werner‟s theory of coordination compounds, V.B. and M.O. theory of bonding in metal complexes, electronic spectrum, magnetic and spectral properties of metal complexes. Organometallic compounds. Bioinorganic chemistry, the biological role of alkaline earth metal ions, metalloporphyrins. Analytical Chemistry: Principles and methods of chemical analysis, principles involved in separation techniques, chromatography |
9 | Modern concepts of covalent bonding, bond lengths, energy and bond angles, electron displacements, inductive, electromeric, mesomeric and hyper conjugative effects, resonance ad its applications to organic chemistry, tautomerism, effects of structure on chemical reactions, dissociation constants. Mechanism of organic reactions: Types of reagents and organic reactions, reaction intermediates, product analysis, isotope effects, kinetic and stereochemical studies. 55 Stereochemistry: Optical and geometrical isomerism, chirality, enantiomers, stereogenic centres, diastereomers, resolution and racemization, relative and absolute configuration, sequence rules, E&Z and R&S nomenclature, the concept of conformation and conformational analysis of ethane, butane and cyclohexane and sugars. Chemistry and reactions of derivatives of aliphatic and aromatic compounds including Alkanes, alkynes and alkenes. arenes and aromaticity, benzene and polynuclear hydrocarbons, alkyl and aryl halides, SN1, SN2 and SNi reactions, nuclear and side-chain reactions, aromatic substitutions reactions, elimination reactions. Aliphatic and aromatic alcohols and phenols, ethers and epoxides, aliphatic and aromatic aldehydes and ketones, aliphatic and aromatic carboxylic acids and their derivatives, aliphatic and aromatic amines and amides, synthetic applications of diazonium salts. Amino-acids. Reactions and applications of organometallic compounds, Acetoacetic and malonic esters, Organic synthesis via enolates. Heterocyclic compounds, pyridine, quinoline, thiophene, furan and pyrrole. Important organic name reactions and rearrangements of synthetic importance. Carbohydrates, classification and general reactions, glucose, fructose and amino acids and proteins, terpenoids and alkaloids. Polymers, dyes and pigment. Theory and application of spectral techniques, UV, IR and NMR in structure elucidation of simple organic molecules |
10 | Elementary quantum mechanics Gaseous states: Kinetic theory of gases and gas laws, Maxwell‟s law of distribution of velocities, Van der Waal‟s equation, Law of corresponding states, Liquefaction of gases, Ratio of Cp/CV. Thermodynamics: The first law of thermodynamics, Isothermal and adiabatic expansion, Enthalpy, heat capacities, Thermochemistry –heats of reaction, formation, solution and combustion, Calculation of bond energies, Kirchhoff equation, Criteria for spontaneous changes, the second law of thermodynamics entropy. Free energy, criteria of thermodynamic equilibrium. Solutions: osmotic pressure lowering of vapour pressure, depression of freezing point, the elevation of boiling point, determination of molecular weights, association and dissociation of solutes. Chemical equilibrium, the law of mass action and its applications to homogeneous and heterogeneous equilibrium, Le Chatelier‟s principle, Influence of temperature on chemical equilibrium. Electrochemistry: Faraday‟s laws of electrolysis, the conductivity of an electrolyte: equivalent conductivity and its variation with dilution, the solubility of sparingly soluble salts, electrolytic dissociation, Ostwald‟s dilution law, the anomaly of strong electrolytes, solubility product, the strength of acids and bases: hydrolysis of salts, hydrogen ion concentration buffer action, the theory of indicators. Electrochemical cells: Reversible cells, standard hydrogen and calomel electrodes and redox-potentials, concentration cells, determination of pH transport number and ionic product of water, Potentiometric titration, chemical kinetics: Molecularity and order of a reaction, First-order and second-order reactions, Determination of order of a reaction, temperature coefficients and energy of activation, collision theory of reaction rates, Activated complex theory. Phase rule: Explanation of the terms involved, Applications to one and two-component system, reduced phase rule, distribution law. Colloids: General nature of colloidal solutions and their classification, general methods of preparation and properties of colloids, coagulation, protective action, gold number, adsorption phenomenon and adsorption isotherms. Catalysis: Homogeneous and heterogeneous catalysis, catalytic promoters and poisons. Photochemistry: Law of photochemistry. Simple numerical problems |
GBPUAT MCA Entrance Exam Syllabus
Candiadtes can check the GBPUAT syllabus 2026 for MCA in the table given below:
Section Name | Topics |
|---|---|
Mental Aptitude |
|
Mathematics |
|
Computer Awareness |
|
| General English |
|
Click here for the latest GBPUAT 2026 PG syllabus!
For all the latest updates regarding the GBPUAT syllabus 2026 and other aspects of the exam, the candidates must stay tuned to CollegeDekho!
Candidates can check the important topics for the UG course.
| Subjects | Sub Topics | Important Topics |
|---|---|---|
| Physics | Mechanics |
|
| Thermodynamics |
| |
| Waves & Sound |
| |
| Electrostatics |
| |
| Current Electricity |
| |
| Magnetism & Electromagnetism |
| |
| Optics |
| |
| Modern Physics |
| |
| Chemistry | Physical Chemistry |
|
| Organic Chemistry |
| |
| Inorganic Chemistry |
| |
| Biology | Botany |
|
| Zoology |
| |
| Agriculture |
|
Want to know more about GBPUAT
Yes, there is a negative marking of one mark and for each correct answer, you get three marks. The GBPUAT 2025 Exam is a total of 600 marks and there are a total of 200 questions.
To prepare for GBPUAT exams, candidates can refer to various prep books including books like "GB Pant University: Combined Pre-Entrance Exam Guide" by RPH Editorial Board, and "GB Pant University of Agriculture and Technology: Entrance Exam Guide" by RPH Editorial Board.
Pantnagar University Entrance Exam Syllabus for PG courses includes Mental Aptitude, Mathematics, Computer awareness and General English.
The GBPUAT exam syllabus includes Mathematics, Botany, Zoology, Chemistry, and Agriculture for UG courses, as well as Life Sciences, Physical Sciences, and MCA for PG courses.
The courses and topics to be prepared for the examination are listed in the GBPUAT syllabus 2025. Therefore, you can anticipate achieving a high score and ranking in the GBPUAT entrance exam 2025 if you master this exam syllabus and the other areas of the test preparation.
Aspirants should be able to rapidly skim the GBPUAT 2025 syllabus to gain an understanding of the subject matter, what they will be studying, who can be reached for help with any area of the curriculum, and what they require to do well on the actual examination.
Typical response between 24-48 hours
Get personalized response
Free of Cost
Access to community