Never Miss an Exam Update
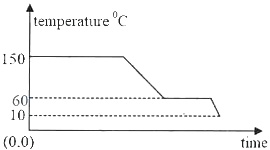
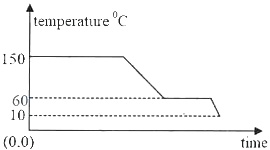
Prepare thoroughly with the most important questions of ICSE Class 10th Science Chapter 11 - Calorimetry. You can first cover the ICSE Class 10th Science syllabus to understand the key topics and then start solving the ICSE Class 10th Science Chapter 11 - Calorimetry Important Question to get a better understanding of your preparation level. Start practicing now.