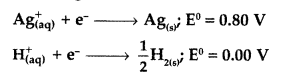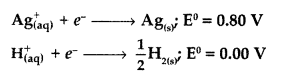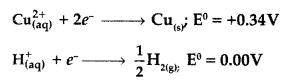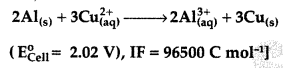Never Miss an Exam Update
Prepare thoroughly with the most important questions of CBSE Class 12th Chemistry Chapter 2 - Electrochemistry. You can first cover the CBSE Class 12th Chemistry syllabus to understand the key topics and then start solving the CBSE Class 12th Chemistry Chapter 2 - Electrochemistry Important Question to get a better understanding of your preparation level. Start practicing now.
Are you feeling lost and unsure about what career path to take after completing 12th standard?
Say goodbye to confusion and hello to a bright future!

Was this article helpful?